Cyber Security News Aggregator
.Cyber Tzar
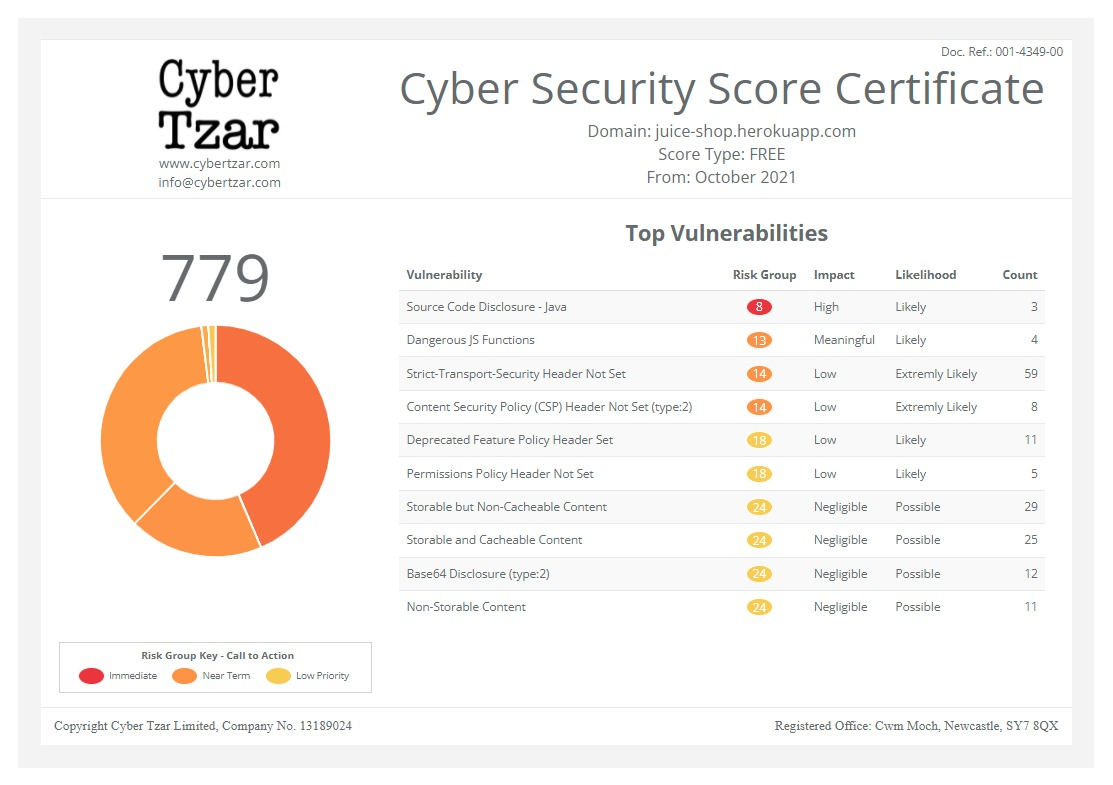
provide acyber security risk management
platform; including automated penetration tests and risk assesments culminating in a "cyber risk score" out of 1,000, just like a credit score.Quickpost: Testing A Lemon Battery
published on 2022-10-22 21:59:06 UTC by Didier StevensContent:
In a chat with my colleagues, we were joking about charging smartphones with a lemon battery.
And I actually wanted to know what magnitude of electrical energy we were talking about.
So I connected a lemon battery to an electronic load:

I took a lemon, inserted a zinc and copper piece of metal (a couple centimeters deep) and connected an electronic load to draw 1 mA of current.
I let it run for a couple of hours until no more measurable current flowed.

The electronic load dissipated 0,034 Wh of electrical energy over that period. Hence, we can assume that the lemon battery delivered 0,034 Wh.
I’m sure the lemon battery could deliver more energy, by “resetting” it: cleaning the electrodes, inserting them in another place in the lemon, …
After a bit of searching through the web, I’m going to assume that a typical smartphone nowadays has a battery of 10 Wh. So we would need 294 times (10 Wh / 0,034 Wh) the electrical energy delivered by my lemon battery to charge a smartphone.
Except that, the 0,9 V that the lemon battery does deliver, is by far not enough to be able to charge via the USB interface. We need 5V, so, 5,555… lemon batteries connected in series.
On the screenshot above, you can also see that 37 mAh was measured. Notice that you can not compare this to the mAh rating of a (smartphone) battery, because both values involve different voltages.
Comparing this to a button cell like a CR2032 (Dutch Wikipedia article, because there’s no English Wikipedia article): the CR2032 has a 225 mAh electrical charge (on average) and a 2.0 discharge voltage. That’s 225 mAh * 2.0 V = 450 mWh. Or 13 times more than my lemon battery (34 mWh).
Here are more pictures of the lemon after the experiment (one week later):




Quickpost info
https://blog.didierstevens.com/2022/10/22/quickpost-testing-a-lemon-battery/
Published: 2022 10 22 21:59:06
Received: 2022 10 22 22:28:42
Feed: Didier Stevens
Source: Didier Stevens
Category: Cyber Security
Topic: Cyber Security
Views: 12